GDX-407填充柱 医用口罩环氧乙烷残留量测定GB/T 16886.7-2015
产品名称:GDX-407填充柱 医用口罩环氧乙烷残留量测定GB/T 16886.7-2015
产品型号:GDX-407填充柱
产品厂商:浩瀚色谱(山东)应用技术开发有限公司
简单介绍
医用口罩环氧乙烷残留量测定GB/T 16886.7-2015,芝麻香白酒,3-甲硫基丙醇,室内空气,焦炉煤气,炼厂气,天然气,变压器油,多氯联苯,植物油,增塑剂,塑化剂,过氧化物
GDX-407填充柱 医用口罩环氧乙烷残留量测定GB/T 16886.7-2015的详细介绍
医用口罩环氧乙烷残留量测定GB/T 16886.7-2015
医用口罩环氧乙烷残留量测定GB/T 16886.7-2015 详细信息:
浩瀚色谱(山东)应用技术开发有限公司参考RB/T 030—2020《化学分析中测量不确定度评估指南》,文中依据GB/T 14233.1—2008《医用输液、输血、注射器具检验方法第1部分:化学分析方法》测定医用口罩中环氧乙烷残留量的检测结果并进行不确定度评定,系统分析了顶空气相色谱法在测定环氧乙烷残留量的不确定度来源,并对各不确定度分量进行量化,并给出方法的合成不确定度。医用口罩中环氧乙烷残留量结果为(9.70±0.60)μg/g,k=2。
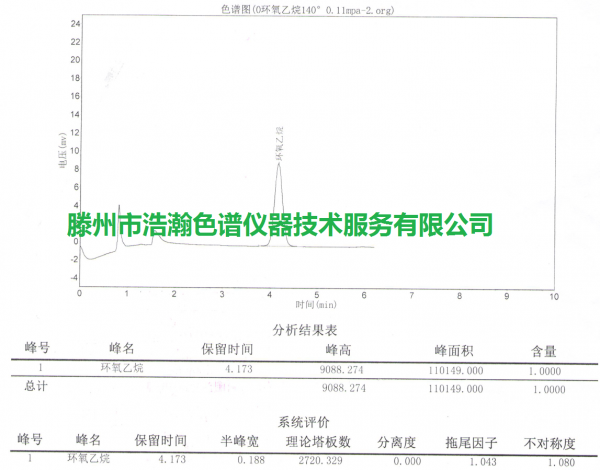
医用口罩环氧乙烷残留量测定GB/T 16886.7-2015 测试谱图:






Determination of ethylene oxide residues in medical masks GB/T 16886.7-2015
Determination of ethylene oxide residues in medical masks GB/T 16886.7-2015 Details:
Name: Packed Column
Statio
Particle size: 80-100 mesh, 60-80 mesh
Specifications: 2m*4mm, 2m*3mm, 2m*1*8
Model: 407 organic carrier, GDX-407
Application: GB/T 16886.7-2015 Biological e
Haohan Chromatography (Shandong) Application Technology Development Co., Ltd. refers to RB/T 030-2020 "Guidelines for the e
Determination of ethylene oxide residues in medical masks GB/T 16886.7-2015 Test spectrum:
医用口罩环氧乙烷残留量测定GB/T 16886.7-2015 详细信息:
名称:填充柱
固定相:有机担体
粒度:80-100目,60-80目
规格:2m*4mm,2m*3mm ,2m*1*8
型号:407有机担体 ,GDX-407
应用: GB/T 16886.7-2015医疗器械生物学评价 第7部分:环氧乙烷灭菌残留量
浩瀚色谱(山东)应用技术开发有限公司参考RB/T 030—2020《化学分析中测量不确定度评估指南》,文中依据GB/T 14233.1—2008《医用输液、输血、注射器具检验方法第1部分:化学分析方法》测定医用口罩中环氧乙烷残留量的检测结果并进行不确定度评定,系统分析了顶空气相色谱法在测定环氧乙烷残留量的不确定度来源,并对各不确定度分量进行量化,并给出方法的合成不确定度。医用口罩中环氧乙烷残留量结果为(9.70±0.60)μg/g,k=2。
医用口罩环氧乙烷残留量测定GB/T 16886.7-2015 测试谱图:







Determination of ethylene oxide residues in medical masks GB/T 16886.7-2015
Determination of ethylene oxide residues in medical masks GB/T 16886.7-2015 Details:
Name: Packed Column
Statio
Particle size: 80-100 mesh, 60-80 mesh
Specifications: 2m*4mm, 2m*3mm, 2m*1*8
Model: 407 organic carrier, GDX-407
Application: GB/T 16886.7-2015 Biological e
Haohan Chromatography (Shandong) Application Technology Development Co., Ltd. refers to RB/T 030-2020 "Guidelines for the e
Determination of ethylene oxide residues in medical masks GB/T 16886.7-2015 Test spectrum:
相关产品
在线留言 |

















